39-year-old, Matthew Moore of North Carolina is now the first U.S. patient with a Carmat total artificial heart (TAH), who received the implant on Monday, July 12 by surgeons Jacob Schroder and Carmelo Milano in an 8-hour long surgery after he had a sudden heart failure. This specific TAH called ‘Aeson’ – made by French company CARMAT mimics the human heart and provides the recipient more independence after the surgery, unlike conventional artificial hearts. Just like a real heart, Aerson has 2 ventricles and 4 valves and is powered by an external device.
Aeson has been approved by FDA for enrollment and has 10 patients enrolled, who are suffering from end-stage biventricular heart failure. It is made from “biocompatible materials”, which includes bovine tissues, along with artificial sensors and algorithms to maintain its function, pace, and to keep the blood circulating.
Matthew Moore was expecting a heart bypass surgery after a sudden unexpected diagnosis of heart failure, but since his conditions kept rapidly deteriorating, so the doctors ran out of traditional options, but he was lucky that Duke was among just three transplant centers in the United States selected to join the device study, and the procedure team received specialized training to prepare for the implant surgery.
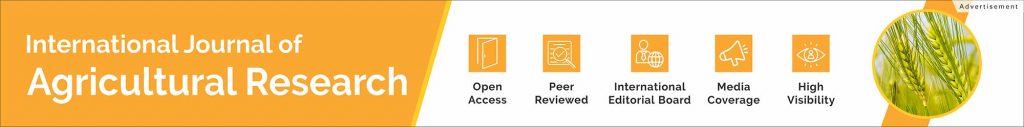
Milano, the transplant surgeon and principal investigator of the device at Duke said that “We are encouraged that our patient is doing so well after the procedure Monday. As we evaluate this device, we are both excited and hopeful that patients who otherwise have few to no options could have a lifeline.”
The Aeson device is made to help patients with biventricular heart failure, as a part of a short-term treatment solution, before a patient can receive a heart transplant, termed to bridge to transplantation. There has been a surge of cases of hospitalization secondary to methamphetamine-related heart failure (HF). Since the availability of the heart may take months, because of the increased demand for a heart transplant, so the team at Carmat are now testing Aeson for up to 180 days to 6 months of use, to give the patient with heart failure a ray of hope and extra time to find a transplant.
Previously, Ventricular assist devices (VADs) and total artificial hearts are often used temporarily to help a failing heart while the patient is waiting for a transplant (“bridge to transplant”). Each patient has one of these devices is entered in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), to allow physicians to determine whether artificial devices could be a safer and effective alternative to human heat, by analyzing the information gathered by such database.
Out of the 10 enrolled patients, the first 3 patients will be monitored for 60 days post surgeries, and then the other 7 patients will be given the implant. And after French Government has given funding to the company to conduct a 52-person clinical trial of Aeson, it has been made commercially available in Europe.














Add comment